
Free Clinical Trial Templates Smartsheet - These trials may supplement earlier trials, complete earlier trials, or may. However, others may also find this template. Phase 2 or 3 clinical trials that require. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. The protocol is the backbone of your clinical trial, detailing every step of. You should also read this: Template For Vows

Free Clinical Trial Templates Smartsheet - This template is adapted from the ich guidance document e6 (good clinical practices), section 6. Its use will also help. Investigators for such trials are strongly encouraged to use this template when developing protocols for nih supported clinical trial(s). However, others may also find this template. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization. You should also read this: Free Printable Letter Templates From Santa

Medical Protocol Template - Background medical students face highly competitive stressful situations throughout their curriculum, which can lead to elevated stress levels and a major decline in quality of life,. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with both. All interventional studies excluding studies. The electronic protocol writing tool aims to facilitate the development. You should also read this: Squarespace Template Timeline
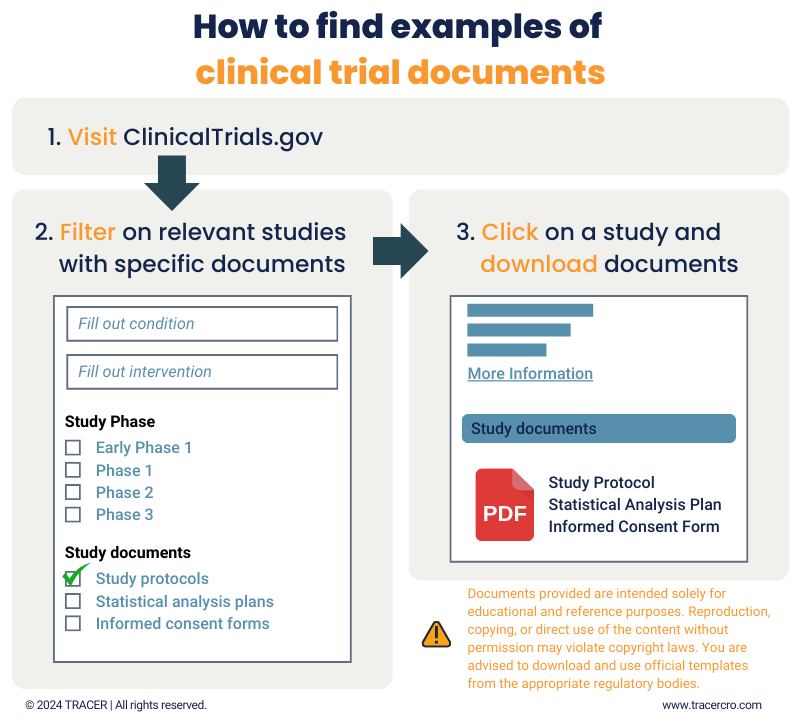
Clinical trial protocol template and example to download TRACER - Prospective data and/or sample collection 3. Please use this template for the following study types: However, others may also find this template. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. The protocol is the backbone of your clinical trial, detailing every step of the study. You should also read this: Thanksgiving Menu Template

Template Device protocol - Clinical trial protocol cqge031c2303 / nct03580356. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with both. Cfaam, bgp, fgs, dsmp, and pmr conceived the idea and study design. Please use this template for the following study types: This template is adapted from the ich guidance document e6 (good clinical practices), section. You should also read this: Year In Review Template Free

Standard Protocol Template NIHR Clinical Research Network - After reading, you will understand how to find a relevant clinical. Welcome to global health trials' tools and templates library. Cfaam wrote the draft version. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with both. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is. You should also read this: Sunshine Template

Clinical Research Protocol Template Edit Online & Download Example - There are two templates to be used for interventional research: Prospective data and/or sample collection 3. This template is adapted from the ich guidance document e6 (good clinical practices), section 6. The template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document. However, others may also find this template. You should also read this: Work Note From Doctor Template

Medical Protocol Template Master of Documents - Prospective data and/or sample collection 3. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically designed for the. This template is adapted from the ich guidance document e6 (good clinical practices), section 6. After reading, you will understand how to find a relevant clinical. The. You should also read this: Elevate Your Design Projects With Psd Templates Graphicpot

Free Protocol Templates to Edit Online & Print - There are two templates to be used for interventional research: Please use this template for the following study types: Cfaam wrote the draft version. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with both. Please note that this page has been updated for 2015 following a quality check and review of. You should also read this: Md Referral Letter Template

Clinical Trial Protocol For Public Reference!!! Clinical Trial - Its use will also help. Cfaam, bgp, fgs, dsmp, and pmr conceived the idea and study design. Clinical trial protocol cqge031c2303 / nct03580356. Prospective data and/or sample collection 3. Fgs provided statistical expertise in clinical trial design. You should also read this: Fiesta Border Template Free