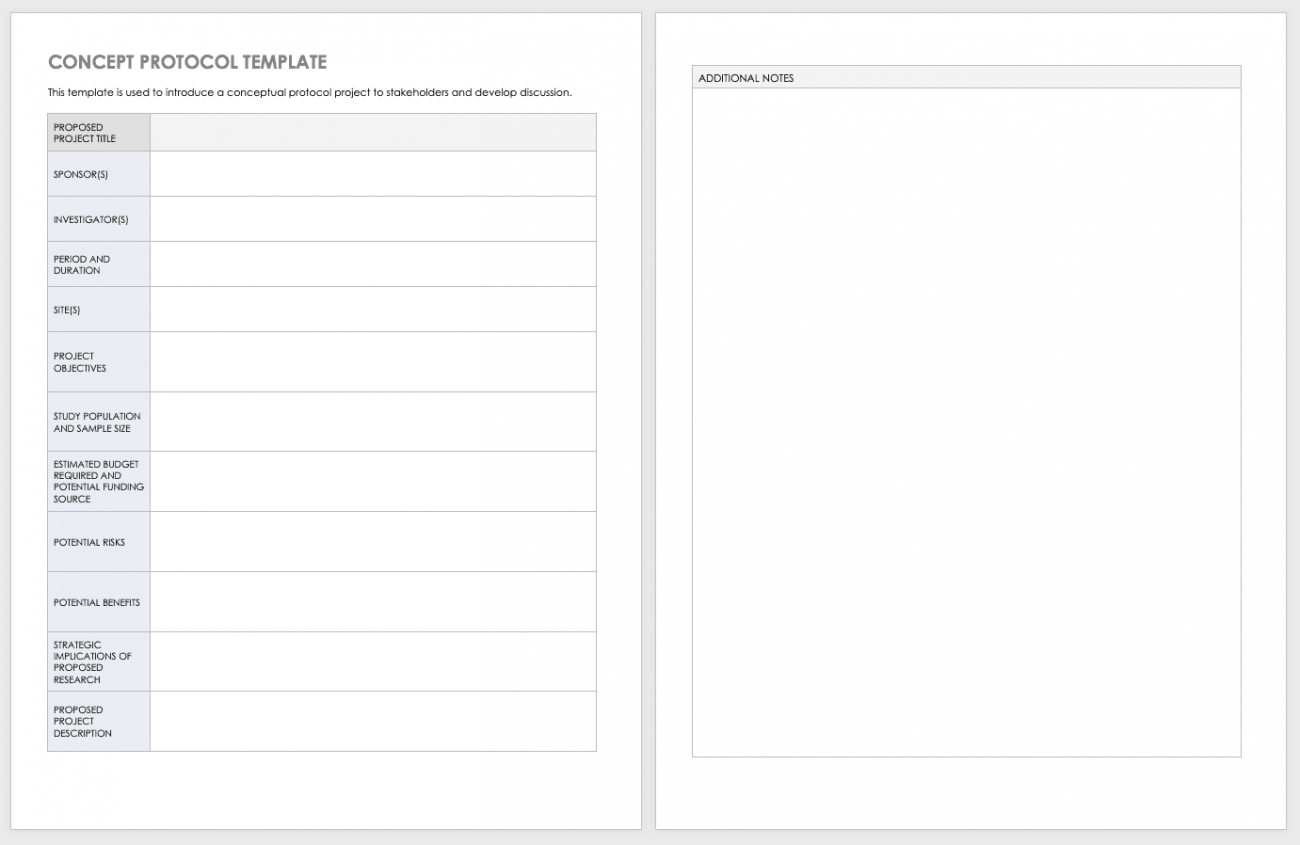
Free Clinical Trial Templates Smartsheet - Send due date reminders using the work progress tracker template with automation features This page includes seven different protocol templates for developing a variety of different new research protocols. None of the templates are likely to be perfect for a given. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies.. You should also read this: Template Devilbreakernavvisual

Sample Research Protocol PDF Clinical Trial Risk - The protocol templates contain instructions and example text that can be included in your protocol. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Site capabilities highlight frequently used resources; Background improving the quality of patient care remains a global necessity. Table 2 of the third. You should also read this: Sign Up Sheet For Potluck Template

Clinical Project Manager Cover Letter Template in Google Docs, Word - In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. This template helps you embrace flexibility and promote collaboration with all stakeholders. The mop is developed to facilitate consistency in protocol implementation and data. Share team news and announcements; Table 2 of the third schedule (page. You should also read this: Indesign Templates Portfolio

Clinical Protocol Template Master of Documents - Despite system and professional benefits, current evidence indicates that the spread of. Table 2 of the third schedule (page. This template helps you embrace flexibility and promote collaboration with all stakeholders. Phase 2 or 3 clinical trials that require. After reading, you will understand how to find a relevant clinical. You should also read this: Burgundy Flower Invitation Template

Minimal Risk Protocol Template INSTRUCTIONS - Use this template to develop your own clinical trial timeline. This template helps you embrace flexibility and promote collaboration with all stakeholders. Provided below are standard templates that can be used by researchers to develop and design their study protocol: Despite system and professional benefits, current evidence indicates that the spread of. The mop is developed to facilitate consistency in. You should also read this: Christmas Window Templates

Free Clinical Trial Templates Smartsheet - Site capabilities highlight frequently used resources; None of the templates are likely to be perfect for a given. This template helps you embrace flexibility and promote collaboration with all stakeholders. Use this template to develop your own clinical trial timeline. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. You should also read this: Offer Letter For Internship Template
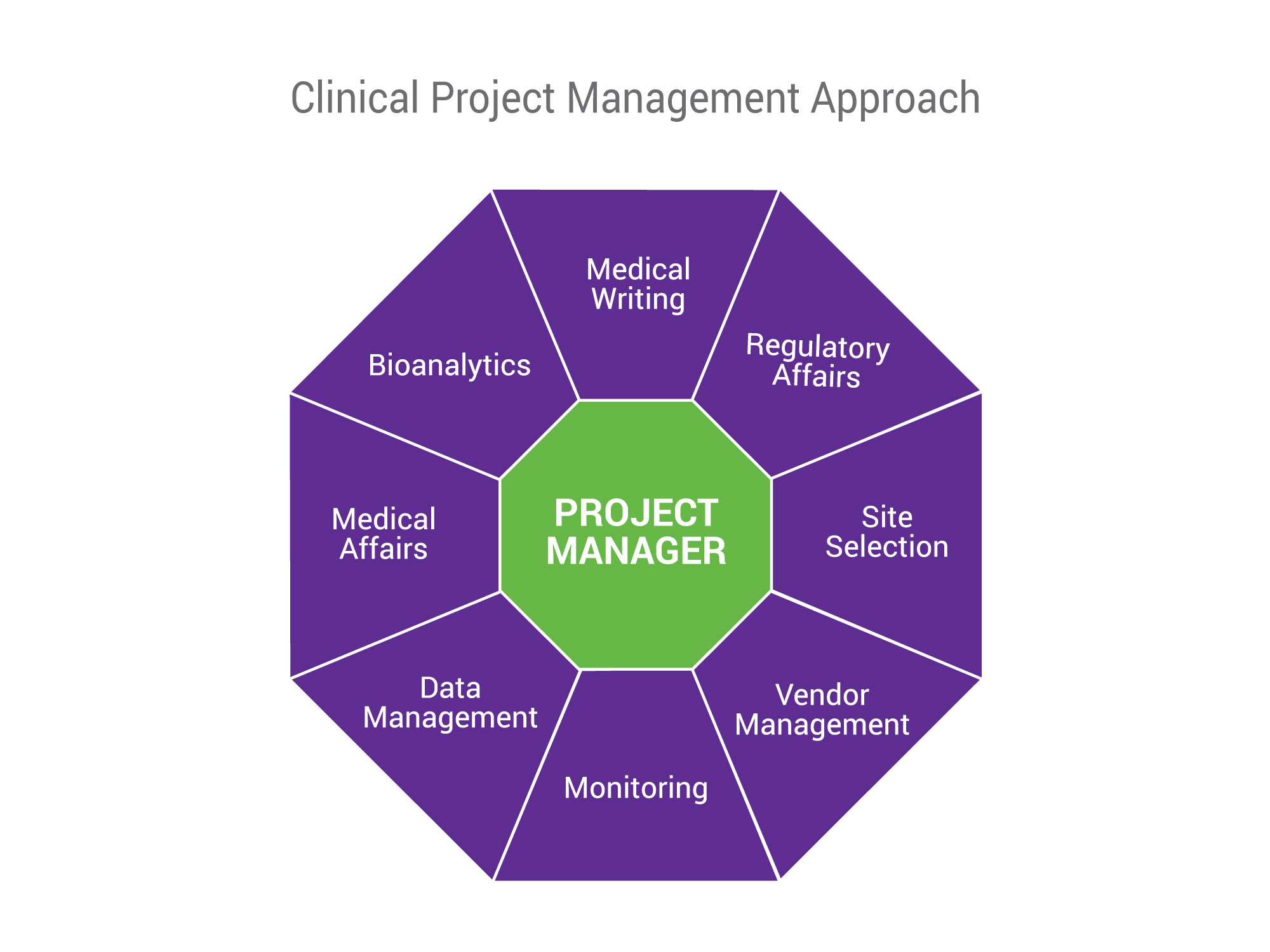
Clinical Project Management Nutrasource - This page includes seven different protocol templates for developing a variety of different new research protocols. Use this template to develop your own clinical trial timeline. However, others may also find this template. Despite system and professional benefits, current evidence indicates that the spread of. Add your own steps, milestones, and dates for a comprehensive, expansive view. You should also read this: Getting To Know Your Teacher Template

Clinical Trial Project Management Plan Template - Welcome to global health trials' tools and templates library. None of the templates are likely to be perfect for a given. It's therefore important for a project manager to develop a realistic. The protocol templates contain instructions and example text that can be included in your protocol. Clinical trial protocol template this protocol template is designed to help research teams. You should also read this: Succession Planning Templates

Clinical Trial Project Management Plan Template - Use this template to develop your own clinical trial timeline. The protocol templates contain instructions and example text that can be included in your protocol. Step 2 of the ich harmonized guideline on the clinical electronic structured harmonized protocol (cesharp) occurred in september 2022. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical. You should also read this: Gum Drop Template

Clinical Study Protocol Template - Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: After reading, you will understand how to find a relevant clinical. Site capabilities highlight frequently. You should also read this: Modeling Resume Template