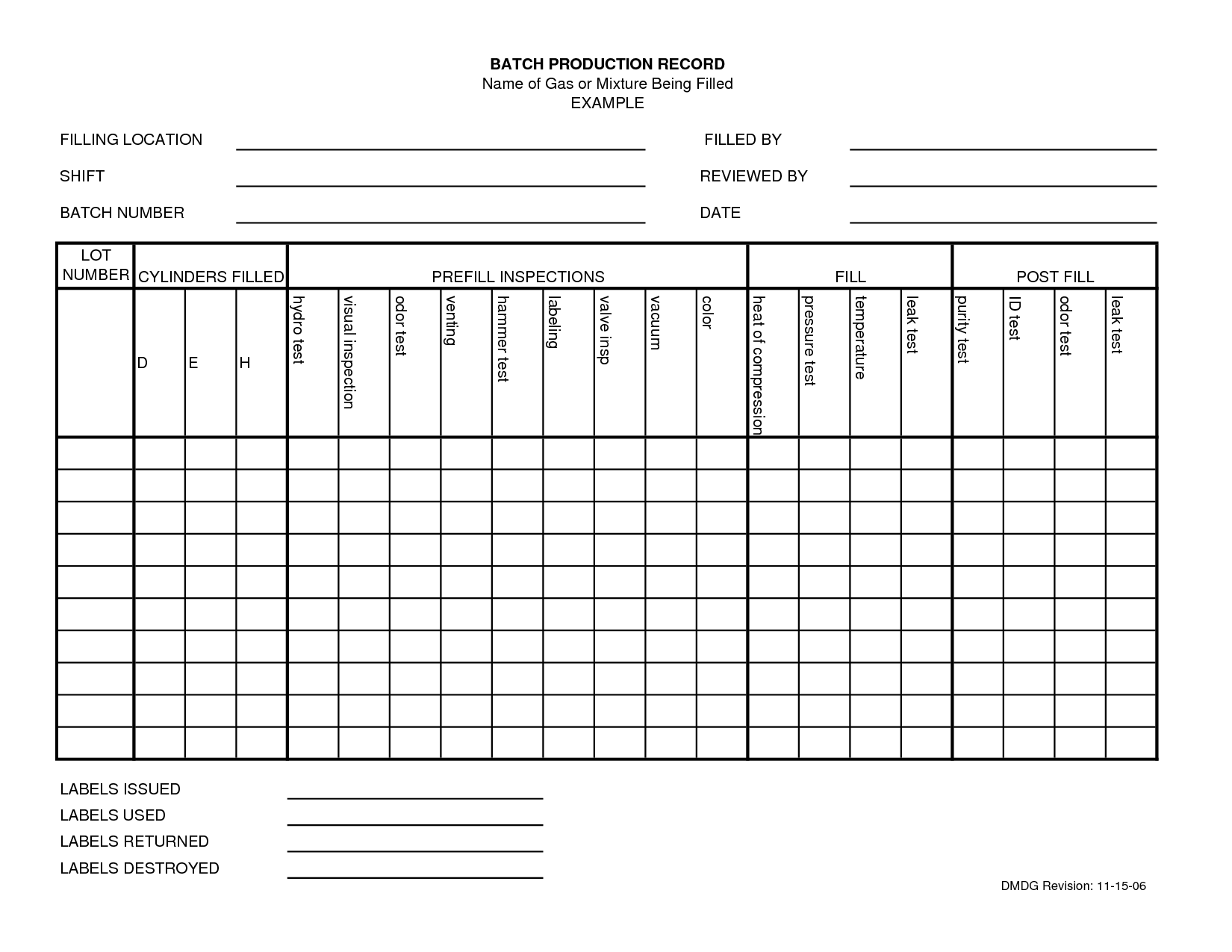
Batch Sheet Template - I have a batch record template i can share. Bmr is specific to a manufacturing location, batch size; The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. Master batch record, probably refers (i say prob cause not a term i am familiar with) to the controlled copy. You should also read this: Polaroid Collage Template

Batch Record Review Checklist Template/Example - That is what i would have said. The device master record should list all of the documents and procedures used to make the product. The device history record is usually a folder that contains (at least in our medical device plant): I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per. You should also read this: Tailwind Blog Template
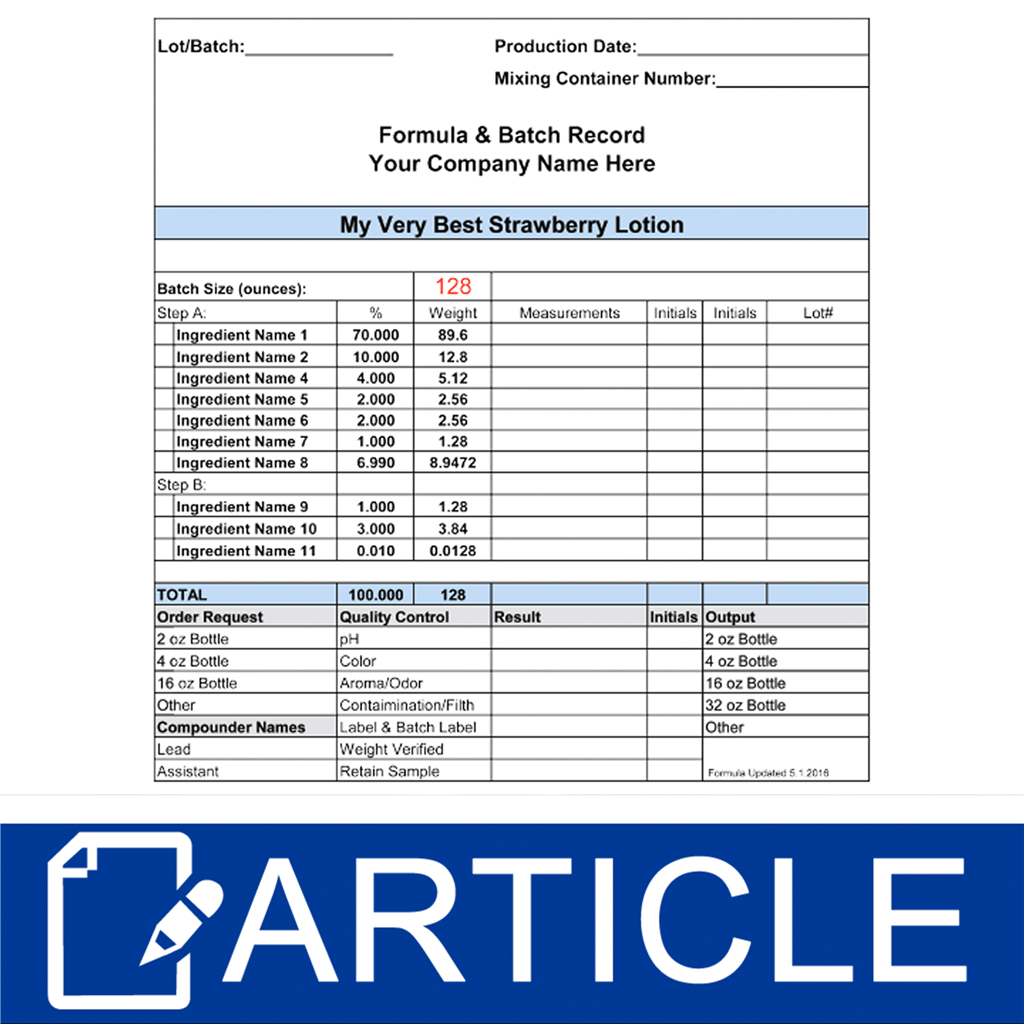
Formula & Batch Records Wholesale Supplies Plus - Qa compliance 10th november 2010 10:32 am re: These requirements are defined in 21cfr 820.3(i) and 21cfr 820.86. Master batch record, probably refers (i say prob cause not a term i am familiar with) to the controlled copy of a batch record held in an area that copies are made from for use when you manufacture, when there is an. You should also read this: Hoa Proposal Template

Good Manufacturing Practices Formula & Batch Records - If any one can guide or discuss with me the batch number allocation system of their manufacturing unit for injection molding department, will b e highly appreciated. Qa compliance 10th november 2010 10:32 am re: The device master record should list all of the documents and procedures used to make the product. That is what i would have said. I. You should also read this: Powerpoint Templates For Birthday Presentations
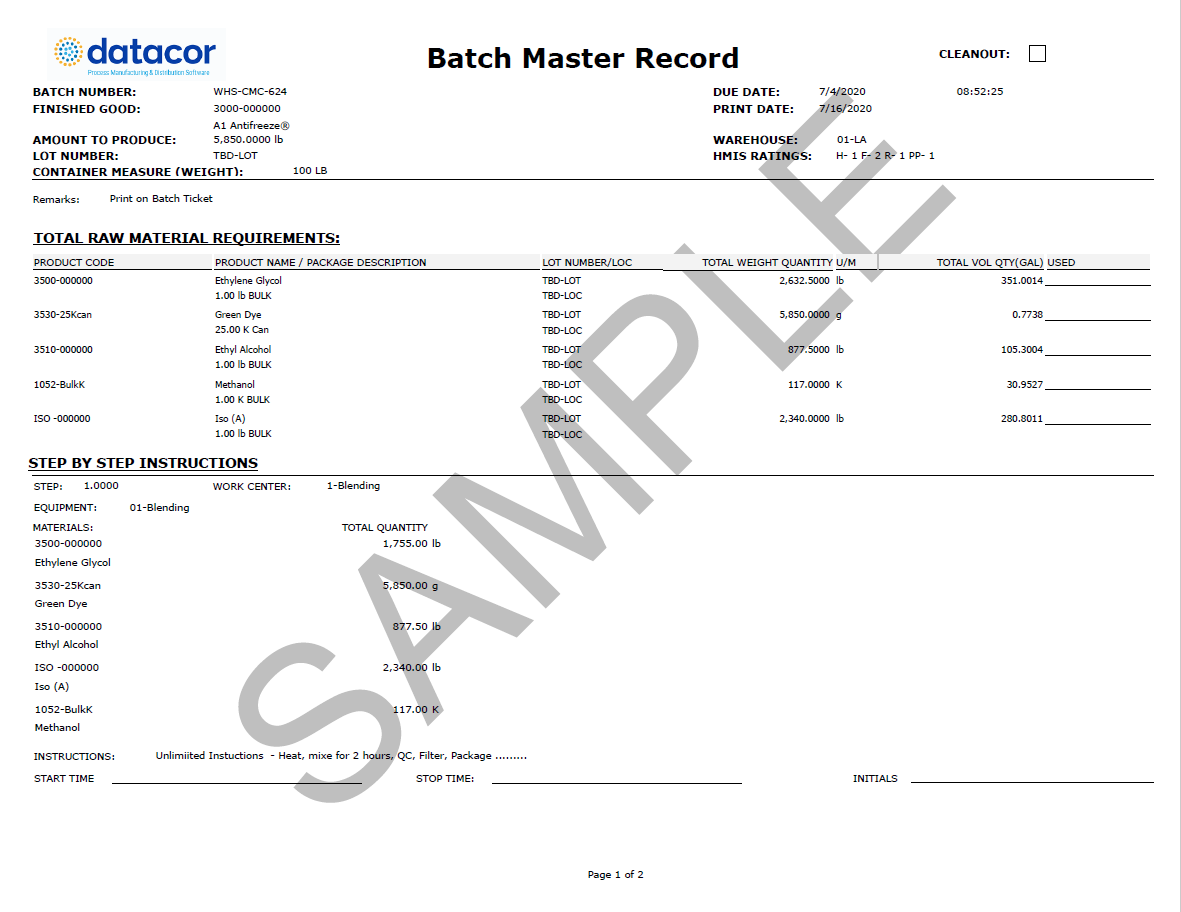
2024 Batch Manufacturing Record Definition, Template & Examples - These requirements are defined in 21cfr 820.3(i) and 21cfr 820.86. Bmr is specific to a manufacturing location, batch size; I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). The device history record is the evidence that a particular unit, batch or lot of devices was made. You should also read this: Free Escape Room Invitation Template

Sample of Batch Manufacturing Record (BMR) Atorvastatin PDF - The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. Master batch record, probably refers (i say prob cause not a term i am familiar with) to the controlled copy of a batch record held in an area that copies are made from for use when you manufacture,. You should also read this: Best Asana Templates

Batch Production Record Templates - I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). Jun 3, 2011 #2 vxmartinez said: It derived based on the master formula record. The device history record is the evidence that a particular unit, batch or lot of devices was made according to the recipe. That. You should also read this: Minion Coloring Template
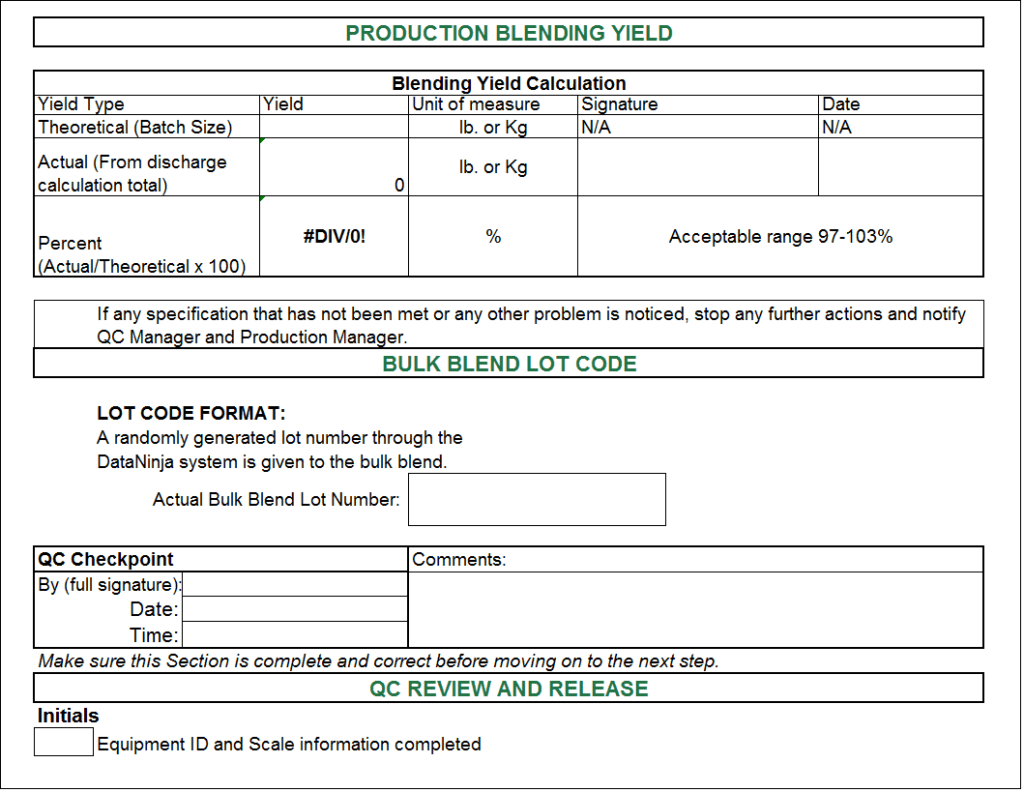
Batch Record Template Excel - If any one can guide or discuss with me the batch number allocation system of their manufacturing unit for injection molding department, will b e highly appreciated. Further i want some guidance in designing batch manufacturing / processing record for injection molding machines for different parts used for syringe manufacturing. These requirements are defined in 21cfr 820.3(i) and 21cfr 820.86.. You should also read this: Fish Outline Template
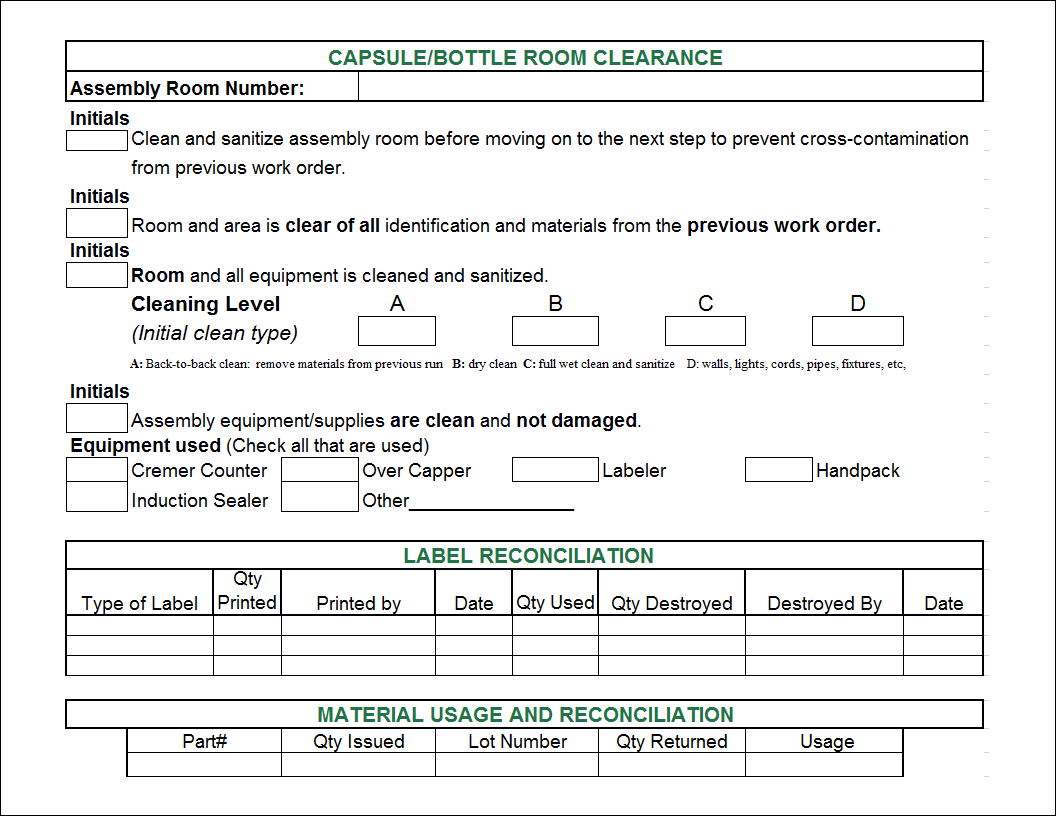
Master Batch Record Template - The device master record should list all of the documents and procedures used to make the product. It derived based on the master formula record. * either a copy of the documents the product was made to or a traveler that lists the documents, revisions, and dates of manufacture. Bmr is specific to a manufacturing location, batch size; These requirements. You should also read this: Turkey In Disguise Project Template
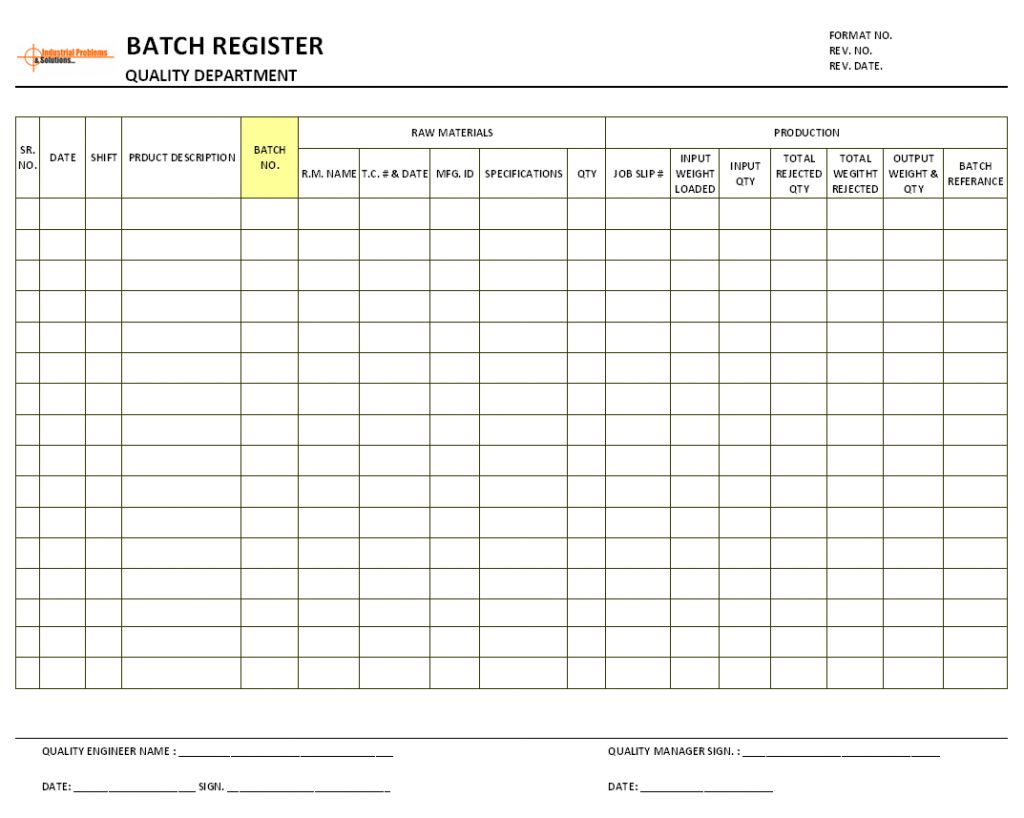
Batch Sheet Template - The device history record is usually a folder that contains (at least in our medical device plant): The device master record should list all of the documents and procedures used to make the product. * either a copy of the documents the product was made to or a traveler that lists the documents, revisions, and dates of manufacture. These requirements. You should also read this: Staff Roster Template Excel